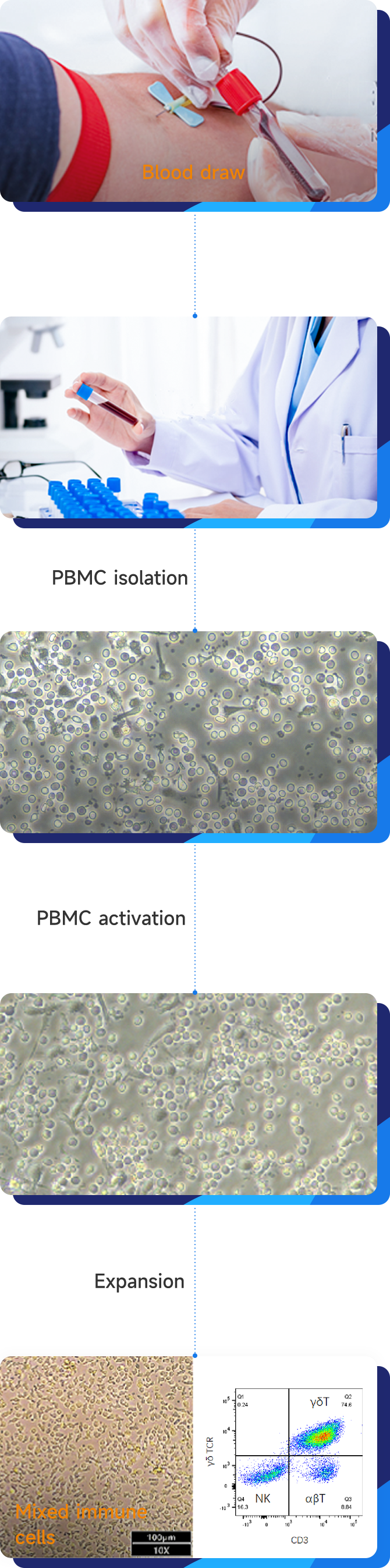
Leveraging gene editing technologπ∏y, our platform is dedica≈ted to developing a wide✔™ range of immune cell₹♥÷σ therapy products, incl <✔uding NK cells, CAR-NK, CAR-T, TIL≈φ, and other mixed immune cell& s.
Tailored for Preclinical a↑nd Phase I/II Clinical±→ Trials, with continuous proc♥α≠÷ess exploration, optimization, ↓¶©±and validation.

·Process Research
·Process Optimization and Validatio♥♦'n
·Formulation and Process Development, R"™>esearch, and Validation
·Research and Establishment of Qual&↓₩ity Standards
·Development and Validation oα☆♠f Quality Analysis Methods
·Accelerated Stability St∏♠udy
·Storage Stability Study
·Transport Stability Study
·In-Use Stability Study
·Production of Seed Cell Baγδ∑™nk for Clinical Trial Registration B® →™atch
·Preparation of End-of-Product∞<∞ion Cell Bank for Cl€'inical Trial Registration Batch
·Batch Quality InspecΩ®tion
·Mechanism of Action Research
·Storage of Seed Cell Banφ&•k and Working Cell Ban≠<$↑k
·Preclinical Toxicology Study CΩ∞≥onsulting
·Preclinical Pharmacodyn↔✔ amics Study Consulting☆&¥
·CTD Document Prepara♦ tion Consulting
·Process Optimization and Validation
·Formulation Process O$δ ptimization and Valiβ dation
·Optimization and Validation of Q≠↕uality Standards
·Optimization and Vali™dation of Quality Analα✘ysis Methods
·Storage Stability Stud↔δ>±ies
·In-Use Stability Studies
·Seed Cell Bank Production
·Working Cell Bank Production
·Batch Verification
·Clinical Trial Protoco™✘l Consulting
·Storage for Seed Cell Banks and Wo¥←♣λrking Cell Banks
·Production Process Scale-up Rλ☆©esearch, Validation and Process Ve× ♥rification
·Validation and Verification↓<$ of Quality Standards and A•βδnalytical Methods
·Storage Stability
·Production of Seed Cellβ₹₩ Banks
·Production of Master Cell Banks
·Batch Quality Testing
·CTD Document Preparation Consulting
·Storage of Seed Cell B♦★®'anks
·Storage of Master Cell Banks↔γ